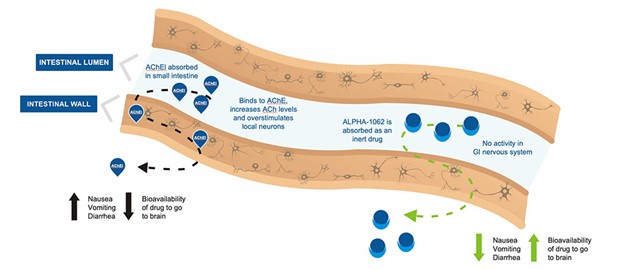
ALPHA-1062 is a prodrug of an approved acetylcholinesterase inhibitor (AChEI), galantamine. It has been uniquely designed to significantly reduce the side-effects observed with the other AChEIs.
ALPHA-1062 is absorbed in the small intestine as an inert drug. Binding with AChE in the gastrointestinal nervous system is blocked by the addition of a benzoyl ester to galantamine. This reduces overstimulation of local neurons, reduces GI side-effects, and increases bioavailability. Once absorbed, ALPHA-1062 is metabolized in the liver to the active drug and galantamine and is carried to the brain in the circulatory system.
AChEIs activity in the GI nervous system overstimulates local neurons resulting in side-effects and decreased bioavailability
The improved side-effect profile of ALPHA-1062 may help to optimize the effectiveness of treatment by enabling patients to start treatment and stay on therapy longer.
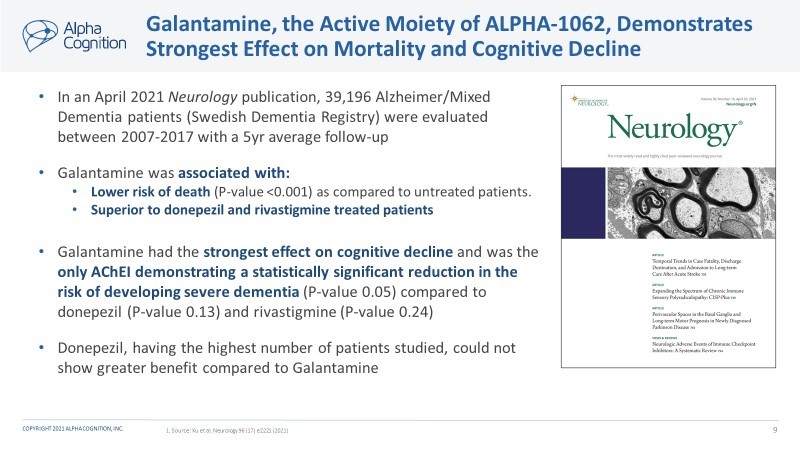
ALPHA-1062 will utilize the 505(b)(2) regulatory pathway for approval in the USA. This approach de-risks the program and accelerates time to launch. Approval for ALPHA-1062 will be based on demonstrating bioequivalence to galantamine. The pivotal program delivered positive results at the end of 2022, and the company plans to file an NDA to the FDA in 2023 with projected approval and launch in 2024.
ALPHA-1062 Potential
- Improved side-effect profile
- Improved bioavailability
- Better Compliance and Adherence
- Optimized effectiveness