A New Approach for the Treatment and Administration of Alzheimer’s Disease
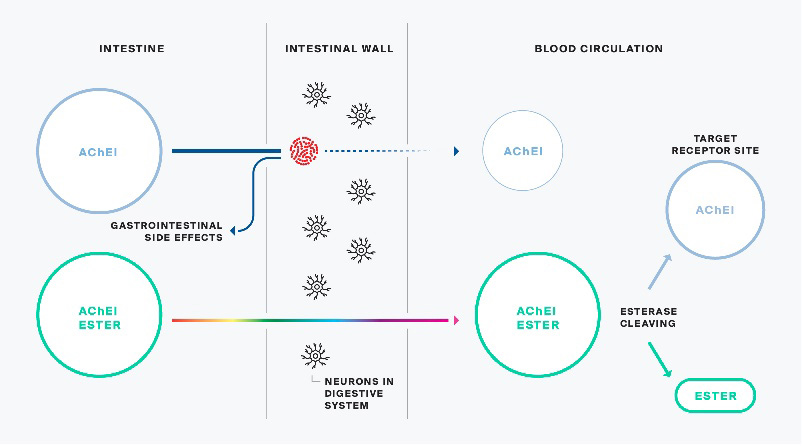
Alpha-1062 is a patented new chemical entity. When ingested, it is enzymatically converted to a US FDA approved drug marketed by Johnson & Johnson as Razadyne in North America, and Reminyl in Europe and elsewhere. Once converted, the active pharmaceutical ingredient of the Johnson & Johnson product called galantamine hydrobromide and Alpha-1062 are identical. However, prior to conversion and during the ingestion process, Alpha-1062 has demonstrated a significantly reduced side effect profile allowing dosing at efficacious levels that may not be achieved by its Johnson & Johnson counterpart. Drugs that convert from an inert form to an active substance in-situ are referred to as “pro-drugs”. At the time the Company licensed the Alpha-1062 technology, only a rudimentary nasal formulation had been developed.
The Company’s Alpha-1062 development plan has two primary goals:
- Clinical Development: Demonstrate to the satisfaction of regulatory bodies that Alpha-1062 formulations have a significantly reduced side effect profile.
- Regulatory Development: Demonstrate that a shortened section 505(b)(2) regulatory path is available to commercialization, utilizing a single short term Pivotal Study.
Alpha-1062 behaves like a Trojan Horse, releasing the active drug into the bloodstream after passing through the intestinal wall as an inert esterified pro-drug.
Clinical Development
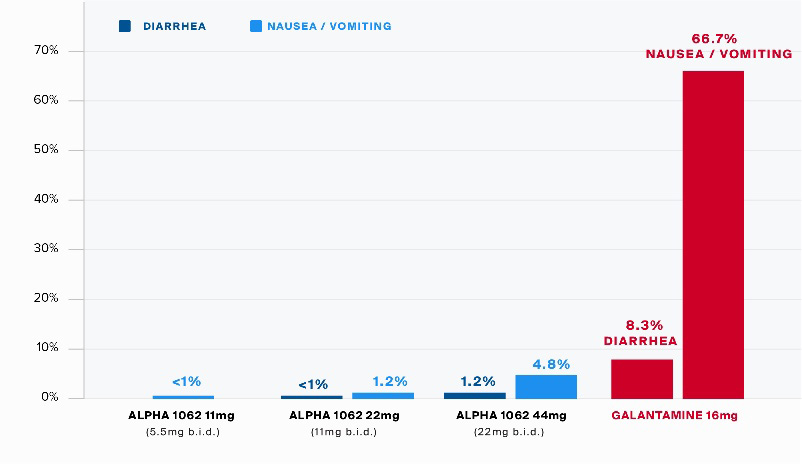
The initial nasal formulation of Alpha-1062 was used to conduct Phase I human toxicology studies, initially by NLS, and subsequently, on completion of the Alpha-1062 Agreement, by the Company. The Phase I human toxicology studies included a single ascending dose trial (“SAD Study”) followed by a multiple ascending dose (“MAD Study”) trial. These Phase 1 studies are designed to determine the safety of the drug, which was administered to healthy patients at increasing doses of Alpha-1062, initially one time in the SAD Study, and subsequently multiple times over a seven-day period in the MAD Study. These studies convincingly demonstrated the Alpha-1062 prodrug formulation had reduced gastrointestinal side effects (nausea, diarrhea, vomiting) by more than 90% as compared to one of the existing treatments, Razadyne.
The Company planned and initiated a series of Pilot Studies in the third quarter of 2020 for the following delivery methods:
- Nasal Spray: Formulations developed for the nasal spray included the selection of the optimal device for nasal delivery. For a nasal spray, the drug and device are independently evaluated by the FDA, and both must meet the FDA’s criteria as a combination drug/device for approval. The Pilot Study will establish the exact spray pattern and dose.
- Sublingual tablet: Data recently collected indicated that Alpha-1062 was well absorbed under the tongue at levels similar to those reported for the reference listed drug, Razadyne. This preliminary data set clearly indicates that an oral dosage is possible and with minimal adjustment of the formulation, can meet clinical trial requirements for approval by the FDA.
- Enteric coated tablet: Data for the enteric coated product, a tablet that is swallowed, when analyzed, will be used to adjust the final dosage to release the prodrug in the small intestine over a period of five to 10 hours. This will allow a product to be taken once-a-day.
Based on the Pilot Study clinical evidence, the Company will select the optimal formulation and delivery mechanism to submit for approval to the FDA. This decision will be taken once the Pilot Study has been completed and the data is reviewed, and a market analysis is completed. At present, the lead product candidates are the sublingual and enteric formulations.
Alpha-1062 subjects were given doses twice per day (“Bis In Die” or “B.I.D.”), over a 7-day period, receiving a total of 168 total doses for each 12-subject cohort.
Summary of Regulatory and Commercial Development
The following is a summary of regulatory steps the Company has planned over the next 24 months:
- Pilot Study: Targeted for completion in the fourth quarter of 2020 with the final report to follow. The study is designed to confirm dosage and format for the Pivotal trial. As designed, it consists of four arms: (a) sublingual tablet, (b) enteric coated tablet, (c) nasal spray, and (d) comparator reference drug, Razadyne. Each arm of the Pilot Study will consist of 10 subjects who will be administered the respective dose of Alpha-1062 and will have blood samples drawn at fixed time intervals. These blood samples will be analyzed to assess blood plasma concentrations of Alpha-1062 and galantamine over time. The validated results will be plotted on a graph showing the initial blood concentrations, which will decline over time. The graph of that particular Alpha-1062 formulation will be compared with the graph of Razadyne, and if required, it will be adjusted for equivalence within 20% variance. The Pilot Study will also allow the Company to determine how many subjects will be required to achieve statistical significance.
- Pivotal Study: Targeted for completion in the second half of 2021. Building on the Pilot Study, the FDA has stated that Alpha-1062 could be approved if the Company completes a single bioavailability/bioequivalence (BABE) clinical trial to confirm that Alpha-1062 is similar to the approved reference listed drug (RLD), Razadyne. The Pivotal Study will be a repeat of the Pilot Study but with more subjects to allow the results to achieve statistical significance. Side effects will also be measured and compared with the RLD.
- New Drug Application: Targeted for the first half of 2022. Following completion of the Pivotal Study, the Company will submit a New Drug Application package for approval to the FDA. The FDA review and approval could be completed within 10 to 12 months following submission.
- Superiority Study: Targeted for 2022. Following the NDA submission, the Company intends to complete a clinical study demonstrating a superior label claim to that of existing treatments by eliminating the traditional up-titration warning, thus allowing immediate efficacious dosing with significantly reduced side effects.
Based on the Pilot Study clinical evidence, the Company will select the optimal formulation and delivery mechanism to submit for approval to the FDA. This decision will be taken once the Pilot Study has been completed and the data is reviewed, and a market analysis is completed. At present, the lead product candidates are the sublingual and enteric formulations.
Commercialization Strategy
Targeted for the second half of 2021 and continuing thereafter, the Company will take steps to develop a commercialization team to manage and monitor product manufacturing and the distribution process. For the international market, the Company intends to identify distribution partners whose sales teams ideally, are focused on pharmaceutical products and therapies for the central nervous system and neuro degenerative diseases. The distribution partners will be licensed to market and sell the Company products, starting with Alpha 1062, world- wide but excluding the United States and certain other countries. The Company will initially focus on identifying distribution partners in smaller countries or territories which will be identified geographically and could consist of one or more regions. At present, the Company intends to find distribution partners in major territories usually identified as Europe, North America, Japan, China. However, to reduce risk, the Company will establish in-house sales and marketing capability for the United States and will likely identify and negotiate marketing partners in the remaining major markets with the goal of finding a single co-marketing partner to drive sales in those major markets. However, in the event a suitable co-marketing partner is not found, the Company will be positioned to launch the product itself.




